With the emergence of investigational gene therapies, patients evaluating options for treatment have a number of important considerations – and likely many questions. Sarepta is committed to educating and empowering our patient communities, and so we spend a good deal of time listening to better understand the questions people may have.
One topic that comes up frequently is the role of steroids in gene therapy. We asked Lisa Borland, PharmD, vice president of Medical Affairs at Sarepta, to help answer this question.
Q: Can you start by explaining how steroids are used in the treatment of neuromuscular conditions?
Lisa: When we talk about steroids in gene therapy, we’re talking about corticosteroids. Corticosteroids are drugs that help to reduce inflammation in the body’s tissue and are used to treat a variety of conditions.1
In some neuromuscular disorders, including Duchenne muscular dystrophy, corticosteroids are prescribed as standard medical care to improve muscle strength and function.2 In other neuromuscular disorders, like limb-girdle muscular dystrophy, corticosteroids are less commonly prescribed as standard of care.3 But all people receiving gene therapy for neuromuscular diseases – including those who are not on corticosteroid treatment as part of their standard of care – are administered corticosteroids before gene therapy dosing.
Q: Why are corticosteroids so important to administration of gene therapy?
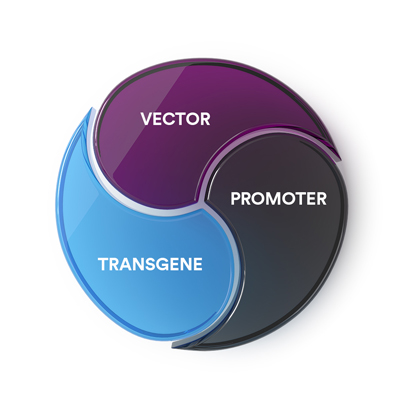
Lisa: To understand the role of corticosteroids in gene therapy, it’s helpful to review the fundamentals of gene therapy. There are three essential components of gene therapy: the vector, the promoter and the transgene.
Quick Facts
- Some gene therapies use a viral vector, or delivery system, to transport corrected or missing genes to cells.
- The body can view that vector as a viral invader and try to fight it.
- Corticosteroids keep the body’s immune system from fighting the gene therapy and interfering with its ability to reach disease-affected cells.
- It is common to give corticosteroids for gene therapy shortly before administration and for a period of time afterwards.
- The physician administering gene therapy treatment will determine how long corticosteroids (or additional corticosteroids) are needed and how to adjust them post-gene therapy.
The vector is the delivery vehicle, responsible for carrying the transgene and the promoter to target cells. The promoter acts like an on/off switch to express protein in the cells. And the transgene acts like an instruction manual, giving the cell instructions for how to make a protein.4
Many investigational gene therapies use a viral vector – an engineered version of a naturally occurring virus – to deliver the promoter and transgene. Viral vectors are the vehicle of choice because viruses are particularly good at gaining access to hard-to-reach cells. The vectors used in gene therapy are designed so they are not harmful to humans, but they are structurally very similar to actual viruses.
Next, it’s important to remember the role the body’s immune system plays in maintaining our health. The immune system helps to defend against substances it sees as harmful or foreign, such as bacteria or viruses. When the immune system detects these invaders, it will try to attack or destroy them.
When a viral vector is introduced, the body may not be able to distinguish the vector from a naturally occurring virus. It may identify the vector as a foreign invader and mount an immune response. And that could interfere with the gene therapy and prevent it from working as intended.
Enter corticosteroids. Corticosteroids decrease inflammation in the body and suppress the body’s immune response.1 Research indicates that corticosteroids administered at the time of gene therapy lower the immune system response so that the body can’t fight the viral vector as an invader.5 And that allows the vector to deliver the promoter and transgene to target cells as intended.
Q: When are corticosteroids for gene therapy administered?
Lisa: It’s common to give corticosteroids shortly before administration of gene therapy and for a brief period afterwards. If corticosteroids were not part of a patient’s standard-of-care treatment plan, the physician will determine when and how to adjust the corticosteroids post-gene therapy. Patients who are on corticosteroid treatment as their standard of care will typically receive additional corticosteroids prior to gene therapy dosing. The physician administering gene therapy treatment will determine how long the additional corticosteroids are needed, and when and how to slowly taper the corticosteroid dose back to the patient’s original plan.
Q: Is the need for corticosteroids unique to gene therapies for neuromuscular disease?
Lisa: No, any potential gene therapy that uses a viral vector needs to consider the immune system response. But corticosteroids are especially important in gene therapy for neuromuscular disease.
Why? How much gene therapy is needed – the amount of the gene therapy dose – depends on the nature of the disease being treated, and how many and what type of cells are affected. In neuromuscular diseases, the therapy needs to target and repair many muscle cells across the entire body.6 In comparison, a gene therapy dose for a disease that affects only the eye has fewer cells to reach and the dose is correspondingly smaller. A larger dose means introducing more viral vector, which could trigger a stronger immune response. That’s why pre-dosing with corticosteroids is particularly important in gene therapy for neuromuscular disorders.
Because corticosteroids are associated with some side effects, including mood changes and negative behavior in some people, all corticosteroid treatment should be overseen by a healthcare specialist. It is important that anyone with questions or concerns about corticosteroid administration during gene therapy talk to a healthcare provider.
Learn more
Learn more about gene therapy for neuromuscular disorders at Duchenne.com/genetherapy.
References
1. Hodgens A, Sharman T. Corticosteroids. NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health. Accessed March 10, 2023. https://www.ncbi.nlm.nih.gov/books/NBK554612/
2. Birnkrant DJ, Bushby K, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018 Mar;17(3):251-267. doi: 10.1016/S1474-4422(18)30024-3.
3. Narayanaswami P, Weiss M, et al. Evidence-based guideline summary: diagnosis and treatment of limb-girdle and distal dystrophies: report of the guideline development subcommittee of the American Academy of Neurology and the practice issues review panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology. 2014 Oct 14;83(16):1453-63. doi: 10.1212/WNL.0000000000000892.
4. What is Gene Therapy? U.S. Food and Drug Administration. Accessed March 10, 2023. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy
5. Sack BK, Herzog RW. Evading the immune response upon in vivo gene therapy with viral vectors. Curr Opin Mol Ther. 2009 Oct;11(5):493-503. PMID: 19806497; PMCID: PMC3584155.
6. Elangkovan N, Dickson G. Gene Therapy for Duchenne Muscular Dystrophy. J Neuromuscul Dis. 2021;8(s2):S303-S316. doi: 10.3233/JND-210678.